
|
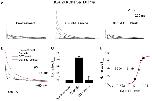
Figure 2. Oxidation by tBHP, a membrane-permeant analog of H2O2, reversibly increases peak current and slows DPP6a-mediated N-type inactivation.A. Representative families of whole-oocyte currents elicited before tBHP treatment, after tBHP treatment, and after subsequent reduction by DTT. Step depolarizations were made from a holding potential of â100 mV to various voltages up to +60 mV in 20 mV increments for 1 sec. B. Superimposed 500-ms-long current traces at +60 mV under the described conditions. Post-treatment trace was scaled to the pre-treatment trace to emphasize the impact on inactivation kinetics. C. Fractional changes in peak current amplitude at +60 mV after tBHP (nâ=â11) and DTT (nâ=â5) treatments. The dashed line represents no-change. D. Concentration dependence of oxidative regulation by tBHP, as measured by normalized peak current amplitude. The rising phase was fitted with a Boltzmann distribution, where the midpoint is the nominal EC50.
|

|
Figure 3. tBHP effects are mediated by slowing the inactivation time course.Biophysical properties of Kv4.2+KChIP3a+DPP6a currents were compared before (black symbols) and after (red symbols) tBHP treatment. A. Voltage dependence of the time point where half of the current is inactivated (t1/2). The difference between the means at +60 mV is significant at pâ=â0.00001. B. Voltage dependence of the time required to reach peak current. pâ=â0.003 for +60 mV values. C. Fractional recovery at â100 mV as a function of interpulse duration in a two-pulse recovery protocol. The curves represent single exponential fits, with time constants that are not significantly different (pâ=â0.178). D. Voltage dependence of steady-state inactivation. Both the midpoint shift and slope change are significant at pâ=â0.042 and pâ=â0.000094, respectively. E. Voltage dependence of relative peak conductance. No significant change was detected, with pâ=â0.076. In both panels D and E, the solid lines represent best fits using first-order Boltzmann functions. See Experimental Procedures for exact recording protocols.
|

|
Figure 4. Oxidative regulation also occurs with DPP10a-mediated N-type inactivation but does not affect inactivation in the presence of DPP6K.A. Superimposed 500-ms-long Kv4.2+KChIP3a+DPP10a current traces at +60 mV before and after tBHP treatment. Post-treatment trace was scaled to pre-treatment trace to show the slowing of inactivation kinetics. B. Quantitation of increased peak current amplitude as observed in (A). The dashed line indicates no-change in amplitude. With tBHP, nâ=â5. Pre-Tâ=â Pre-treatment. C. tBHP slows inactivation of Kv4.2+KChIP3a+DPP10a currents, as measured by t1/2, throughout the voltage range examined. At +60 mV, the difference between means is significant at pâ=â0.05. D. Ternary complex containing DPP6K is insensitive to oxidative regulation by tBHP. Current traces at +60 mV before and after tBHP treatment were overlapped. E. tBHP has no effect on peak current amplitude in the presence of DPP6K. With tBHP, nâ=â4. F. tBHP has no effect on inactivation of ternary channel complex with DPP6K.
|
|
Figure 5. Diamide specifically react with cysteines and produces the same effects as tBHP with similar concentration dependence.A. Outward currents expressed by Kv4.2+KChIP3a+DPP6a channels in response to 1-sec step depolarizations from â100 mV holding potential to +60 mV in 20 mV increments. B. Overlapped 500-ms-long current traces at +60 mV before and after diamide exposure. Post-treatment trace was also scaled to that of pre-treatment trace to illustrate the dramatic slowing of inactivation. C. Relative change in peak current amplitude at +60 mV in response to oxidation by diamide and reduction by DTT. With diamide, nâ=â6; DTT washout, nâ=â3. D. Concentration dependence of oxidative regulation by diamide, as measured by normalized peak current amplitude. The fitted trace is the best fit using a first-order Boltzmann function.
|

|
Figure 6. Cys-13 on DPP6a is the target of both tBHP- and diamide-induced oxidation.A. Kv4.2+KChIP3a+DPP6a/C13S currents in response to 500-ms-long depolarization at +60 mV before and after 1 mM tBHP treatment. Multiple traces are overlapped to show that the current is no longer sensitive to tBHP. B. Response of 500-ms-long Kv4.2+KChIP3a+DPP6a/C13S current at +60 mV to 0.4 mM diamide. Again, overlapped multiple traces show that the mutation C13S renders DPP6a insensitive to diamide. C. Quantitative measurements of change in peak current before and after oxidant treatment. With tBHP, nâ=â3; with diamide, nâ=â7.
|

|
Figure 7. Oxidative regulation does not depend on KChIP3a or specific Kv4 subunit.A. Superimposed 500-ms-long outward current expressed by Kv4.2/Î2-40+DPP6a channels in the presence and absence of 1 mM tBHP. B. tBHP increases peak current amplitude at +60 mV, as seen in (A). With tBHP, nâ=â3. C. t1/2 measurements, showing that tBHP slows inactivation throughout the voltage ranged tested. At +60 mV, difference is statistically significant (pâ=â0.038). D. Superimposed 500-ms-long Kv4.1+KChIP3a+DPP6a current traces at +60 mV before and after tBHP treatment. Substitution of Kv4.1 for Kv4.2 does not alter the ability of tBHP to increase peak current and slow inactivation. E. Quantitation of increased peak current amplitude as observed in (D). With tBHP, nâ=â4. Dashed line represents no increase in current. F. tBHP slows inactivation of Kv4.1+KChIP3a+DPP6a currents over the voltage ranged tested. At +60 mV, the difference is statistically significant, with Pâ=â0.005. Pre-Tâ=â Pre-treatment.
|

|
Figure 8. Cysteine-to-alanine mutations of cysteine residues common to Kv4.2 and Kv4.1.A. Cartoon illustration of the locations of the Kv4.2 intracellular cysteines conserved between Kv4.1 and Kv4.2 (red dots). B. Outward currents expressed by channels formed by cysteine-to-alanine mutants, KChIP3a, and DPP6a at +60 mV before and after exposure to 1 mM tBHP. (left panels) Representative overlapped traces before and after scaling. (middle panel) Quantitation of changes in current amplitude. Data for mutant channel complexes with C320A, C484A, C529A, C530A, and C588A in tBHP were collected in triplicates (nâ=â3). (right panel) Measurement of t1/2 at indicated voltages. S1âS6 â=â transmembrane segments 1 through 6. Nâ=âN-terminus. Câ=âC-terminus.
|

|
Figure 9. Elimination of all Kv4.1 internal cysteines not essential to expression and re-introduction of Cys-322 do not affect oxidative regulation by tBHP.A. Superimposed traces at +60 mV of Kv4.1/C11xA+KChIP3a+DPP6a channels before and after tBHP treatment. B. Increased peak amplitude produced by tBHP observed in (A). With tBHP, nâ=â3. C. Slowing of inactivation kinetics by tBHP indicated in (A), measured as t1/2. At +60 mV, the differences in the mean values are significant (pâ=â0.006). D. Superimposed traces at +60 mV of Kv4.1/C11xA/C322+KChIP3a+DPP6a channels before and after 1 mM tBHP. E. tBHP increases peak current amplitude at +60 mV. With tBHP, nâ=â4. F. tBHP slows inactivation of Kv4.1/C11xA/C322+KChIP3a+DPP6a. Pâ=â0.002 for differences at +60 mV.
|

|
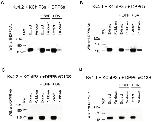
Figure 10. tBHP and diamide do not induce DPP6a gel shift, inconsistent with inter-molecular disulfide bridge.Protein extracts were prepared from oocytes injected with Kv4.2, KChIP3a, and DPP6a cRNAs or with bovine Kv1.4 (bKv1.4) cRNA. Proteins from extracts were separated by SDS-PAGE under reducing and non-reducing conditions, transferred onto Immobilon membranes, and probed with the indicated antibodies. A. DPP6 proteins detected from Kv4.2+KChIP3a+DPP6a protein extracts with or without oxidant treatment using anti-DPP6 antibody. B. bKv1.4 proteins detected from bKv1.4 protein extracts with and without oxidant treatment using anti-Kv1.4 antibody. Results show that DPP6a exhibits no change in migration through SDS-PAGE under non-reducing conditions, while bKv1.4 does. Cntrl â=â Control.
|
|
Figure 11. tBHP and diamide both induce S-glutathionylation of DPP6a in the Kv4 channel complex.A. Western blot loaded with Kv4.2+KChIP3a+DPP6a extract and the pulldown proteins under untreated control, tBHP, and diamide (DA) conditions. B. Western blot loaded with Kv4.1+KChIP3a+DPP6a extract and the pulldown proteins under untreated control, tBHP, and diamide conditions. C. Western blot with Kv4.2+KChIP3a+DPP6a/C13S extract and pulldown proteins under untreated control and oxidized conditions. D. Western blot with Kv4.1+KChIP3a+DPP6a/C13S extract and pulldown proteins under untreated control and oxidized conditions. In all panels, Anti-DPP6 antibody was used to detect DPP6.
|

|
Figure 1. DPP6a and DPP10a N-termini possess a highly conserved cysteine (Cys-13), like other N-type inactivation domains sensitive to redox regulation.A. Alignments of the first 20 residues from the N-termini of pore-forming and auxiliary subunits known to mediate N-type inactivation. Sequences were separated into two groups based on reported redox sensitivity (see Results for reference information). B. Alignments of DPP6a and DPP10a variable N-terminal sequences (20 residues) from various species from human to fish. The unique N-terminal cysteine is highlighted in red.
|











