Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo
Nature. February 14, 2018
Barriga EH , Franze K , Charras G , Mayor R .
Editorial Summary
Tissue mechanics coordinates cell migration in embryos
Groups of cells from distinct germ layers move in an organized fashion to direct morphogenesis and tissue remodelling during embryonic development. Roberto Mayor and colleagues examine the influence of tissue mechanics during the collective migration of neural crest cells in Xenopus laevis. Stiffening of the mesoderm that underlies the neural crest arises as a consequence of a convergent extension movement during gastrulation. Neural crest cells sense the resulting change in the extracellular matrix through integrin signalling, and undergo an epithelial-to-mesenchymal transition before starting their collective migration. This analysis reveals the importance of tissue mechanics in coordinating different events in morphogenesis.
Click here to view article in Nature.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
Collective cell migration is essential for morphogenesis, tissue remodelling and cancer invasion. In vivo, groups of cells move in an orchestrated way through tissues. This movement involves mechanical as well as molecular interactions between cells and their environment. While the role of molecular signals in collective cell migration is comparatively well understood, how tissue mechanics influence collective cell migration in vivo remains unknown. Here we investigated the importance of mechanical cues in the collective migration of the Xenopus laevis neural crest cells, an embryonic cell population whose migratory behaviour has been likened to cancer invasion. We found that, during morphogenesis, the head mesoderm underlying the cephalic neural crest stiffens. This stiffening initiates an epithelial-to-mesenchymal transition in neural crest cells and triggers their collective migration. To detect changes in their mechanical environment, neural crest cells use mechanosensation mediated by the integrin-vinculin-talin complex. By performing mechanical and molecular manipulations, we show that mesoderm stiffening is necessary and sufficient to trigger neural crest migration. Finally, we demonstrate that convergent extension of the mesoderm, which starts during gastrulation, leads to increased mesoderm stiffness by increasing the cell density underneath the neural crest. These results show that convergent extension of the mesoderm has a role as a mechanical coordinator of morphogenesis, and reveal a link between two apparently unconnected processes-gastrulation and neural crest migration-via changes in tissue mechanics. Overall, we demonstrate that changes in substrate stiffness can trigger collective cell migration by promoting epithelial-to-mesenchymal transition in vivo. More broadly, our results raise the idea that tissue mechanics combines with molecular effectors to coordinate morphogenesis.
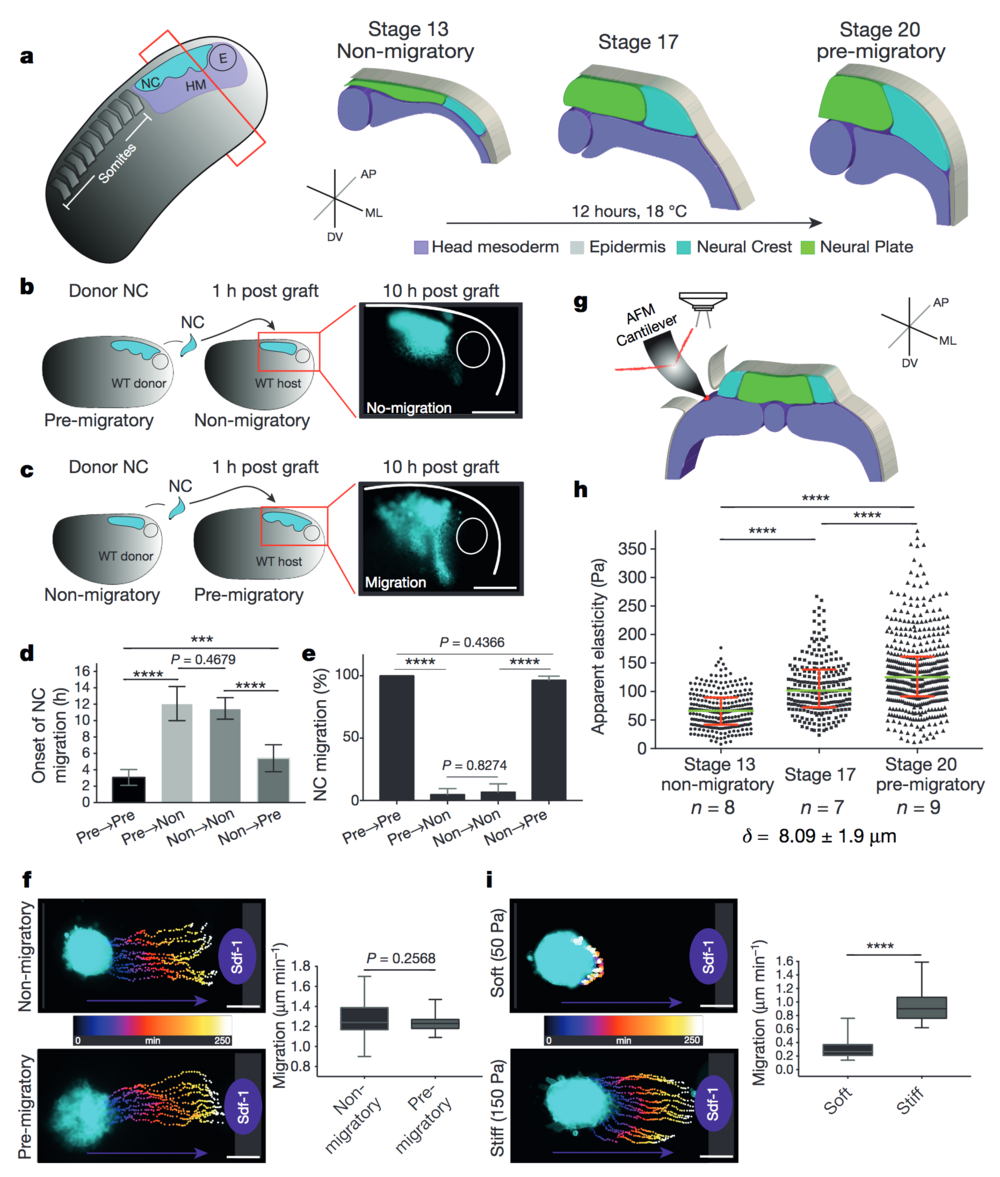
Figure 1. Changes in environmental stiffness are required for neural crest CCM. a, Schematic cross-sections of neural crest development (AP, anteroposterior; ML, mediolateral; DV dorsoventral; NC, neural crest; HM, head mesoderm; E, eye). Cephalic neural crest originates from ectoderm at the border of the neural plate and undergoes EMT before migrating by using head mesoderm as substrate. b–e, Heterochronic grafts. b, Labelled pre-migratory neural crest (cyan) grafted into non-migratory hosts, a representative example of ‘not-migrating’ neural crest at 10 h post-graft is shown. WT, wild-type. c, Non-migratory neural crest (cyan) grafted into pre-migratory hosts, a representative example of ‘migrating’ neural crest at 10 h post-graft is shown. Scale bars, 150 μm (b, c). d, Onset of neural crest migration; x-axis labels indicate migratory status of donor→host. e, Percentage of migrating neural crests at 10 h post-graft. Histograms show mean, error bars represent s.d. (d) or s.e.m. (e); one-way analysis of variance (ANOVA), P < 0.0001; two-tailed t-test, ***P < 0.0002, ****P < 0.0001, CI = 95%, n = 43 embryos in b–e). f, i, Time colour-coded trajectories and quantification of speed of neural crest migration towards Sdf-1. f, Non-migratory versus pre-migratory neural crest. i, Pre-migratory neural crest plated on soft or stiff substrates. Box plots in f and i show the median, box edges represent the 25th and 75th percentiles, and whiskers show spread of data (two-tailed t-test, ****P < 0.0001; n = 78 cells (f); n = 79 cells (i)). g–h, In vivo atomic force microscopy (iAFM) measurements. g, iAFM measurement direct on mesoderm. i, Spread of data for each stage, green lines show median, red whiskers represent interquartile range (two-tailed Mann–Whitney U-test, ****P < 0.0001, CI = 95%; n = 259 (stage 13), n = 236 (stage 17) and n = 461 (stage 20) AFM indentations; n = number of embryos; δ = mean indentation depth). Scale bars, 100 μm (f, i). b, c, f and i show representative examples from three independent experiments; CI = 95%
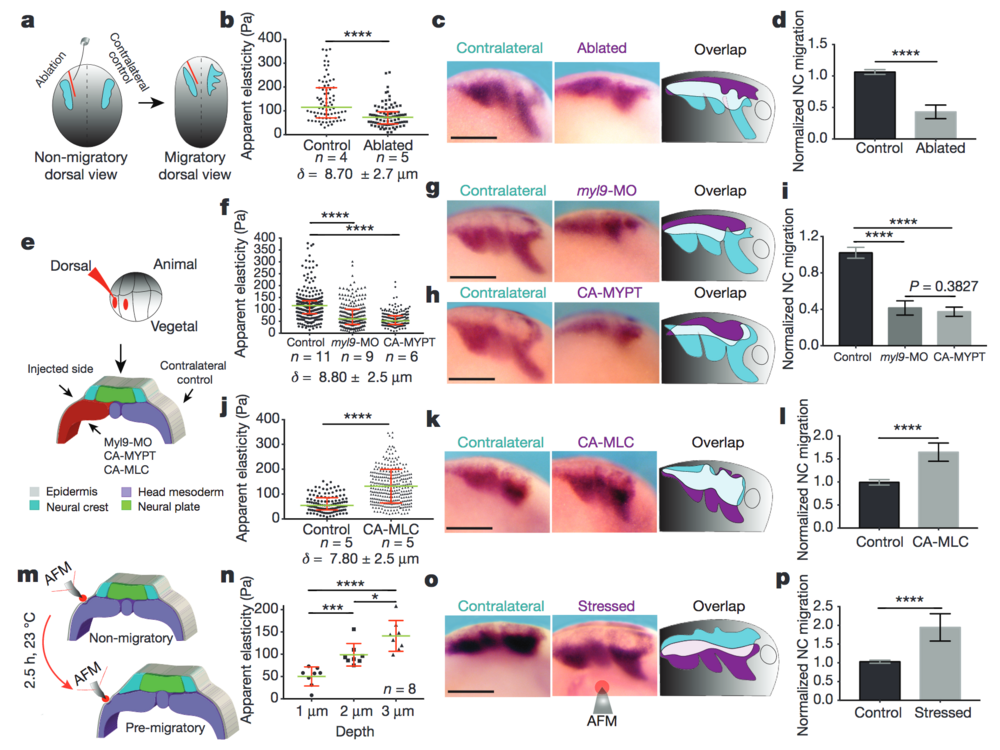
Figure 2: Mesodermal stiffening is essential for neural crest CCM in vivo. a–d, Ablation experiments. a, Schematic showing ablation at non-migratory stages, and neural crest migration at stage 23 (migratory). b, iAFM measurements. n = 78 (control) and n = 86 (ablated) AFM indentations. c, Lateral views of embryos hybridized with a probe against snail2 to analyse neural crest migration. d, Normalized neural crest migration (n = 10 embryos). e–l, Mesoderm-targeted injections. e, Embryos were injected into two dorso-vegetal blastomeres (prospective mesoderm). f, j, iAFM measurements. In f, n = 294 (control), n = 224 (myl9-MO), n = 223 (CA-MYPT); in j, n = 120 (control), n = 301 (CA-MLC). g, h, k, snail2-hybridized embryos. i, l, Normalized neural crest migration. n = 25 embryos (i); n = 12 embryos (l). m–p, Compression experiments. m, Schematic of AFM compression. n, Apparent elasticity plotted as a function of indentation depth (n = 8 embryos), green lines show median and whiskers represent the spread of data (excluding outliers). o, snail2-hybridized embryos. p, Normalized neural crest migration (n = 13 embryos). Overlap drawings in c, g, h, k and o are shown to facilitate comparison of neural crest migration, control neural crest (cyan) and treated neural crest (magenta). b, f, j, Direct iAFM measurements on mesoderm; green lines show median, red whiskers represent interquartile range; two-tailed Mann–Whitney U test, ****P < 0.0001, CI = 95%, n = number of embryos, δ = mean maximum indentation depth. Histograms in d, i, l and p show mean, error bars represent s.d.; one-way ANOVA, P < 0.0001; two-tailed t-test, *P = 0.014, ***P < 0.001, ****P < 0.0001, CI = 95%). c, g, h, k and o show representative examples from three independent experiments. Scale bars, 200 μm.
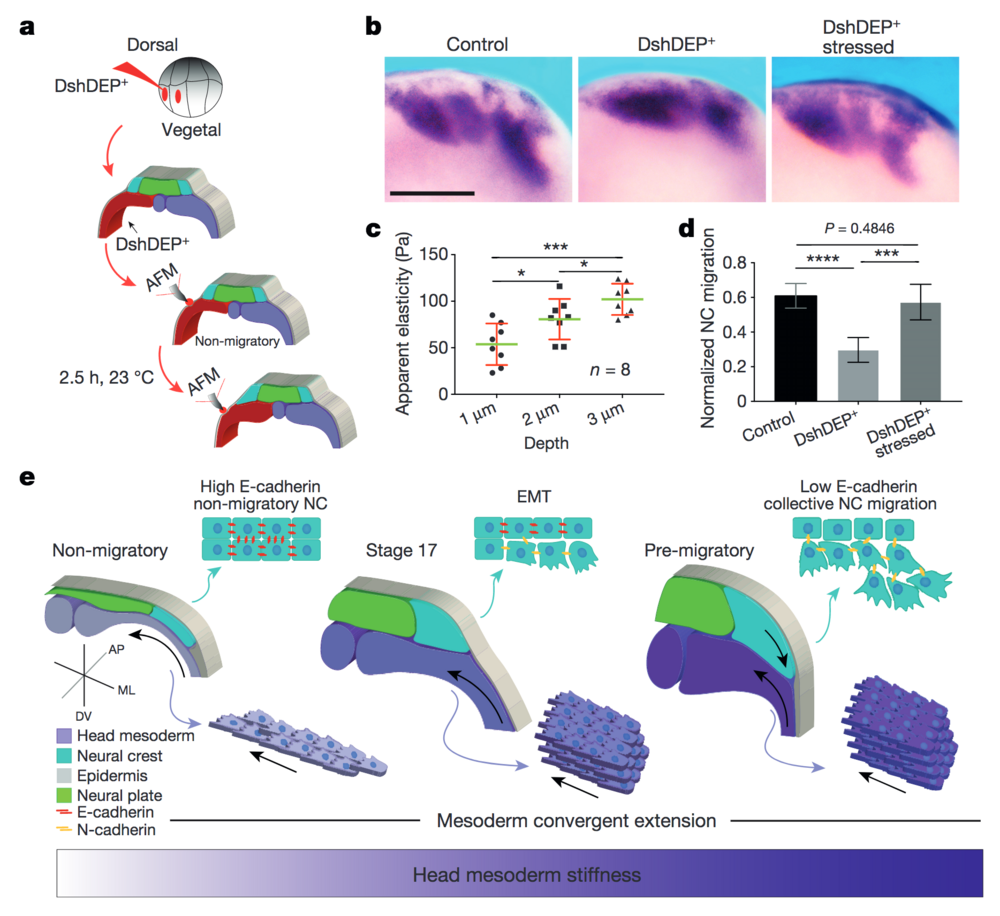
Figure 4: PCP loss-of-function is mechanically rescued by extrinsically inducing mesodermal stiffening. a–d, Mechanical rescue of DshDEP+-induced defects. a, Graphical description of compression experiments. b, Lateral view of snail2-hybridized embryos. Scale bar, 200 μm. c, Induced apparent elasticity plotted as a function of indentation depth. n = 8 embryos; green lines represent median and whiskers show the spread of data (excluding outliers). d, Normalized neural crest migration. n = 19 embryos. b shows representative examples from three independent experiments. Histograms in d show mean and error bars show s.d. In c and d, one-way ANOVA, P < 0.0003; two-tailed t–test, *P < 0.045, ***P < 0.0002, ****P < 0.0001, CI = 95%. e, Schematic representing how mechanical interaction between mesoderm and neural crest coordinates morphogenesis. As convergent extension progresses, the mesoderm stiffens, and in turn, neural crest EMT is triggered and CCM proceeds.
Adapted with permission from Macmillan Publishers Ltd: Barriga et al. (2018). Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature volume 554, pages 523–527 (22 February 2018)
doi:10.1038/nature25742, copyright (2017).
