Notch signaling induces cell death or cell fate change of multiciliated cells
Notch signaling induces either apoptosis or cell fate change in multiciliated cells during mucociliary tissue remodeling
Alexia Tasca, Martin Helmstädter, Magdalena Maria Brislinger, Maximilian Haas, Brian Mitchell, Peter Walentek
Developmental Cell, January 04, 2021 DOI:https://doi.org/10.1016/j.devcel.2020.12.005

Click here to view article at Developmental Cell.
Click here to view article on Pubmed.
Click here to view article on Xenbase
Highlights
- Xenopus multiciliated cells are lost during developmental epidermal remodeling
- Multiciliated cell loss is induced by elevated Notch signaling
- Notch can induce apoptosis or secretory cell fate change in multiciliated cells
- Multiciliated cell cilia loss resembles key aspects of primary cilia retraction
Abstract
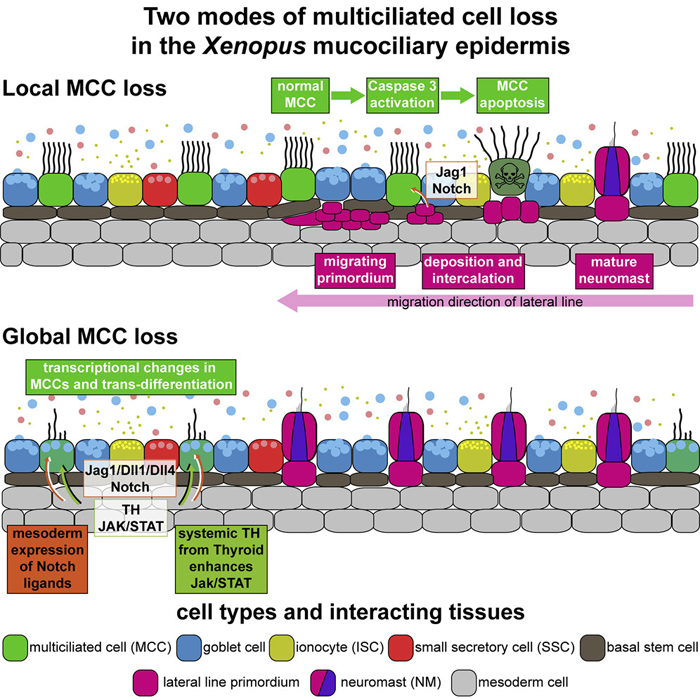
Multiciliated cells (MCCs) are extremely highly differentiated, presenting >100 cilia and basal bodies. Therefore, MCC fate is thought to be terminal and irreversible. We analyzed how MCCs are removed from the airway-like mucociliary Xenopus epidermis during developmental tissue remodeling. We found that a subset of MCCs undergoes lateral line-induced apoptosis, but that the majority coordinately trans-differentiate into goblet secretory cells. Both processes are dependent on Notch signaling, while the cellular response to Notch is modulated by Jak/STAT, thyroid hormone, and mTOR signaling. At the cellular level, trans-differentiation is executed through the loss of ciliary gene expression, including foxj1 and pcm1, altered proteostasis, cilia retraction, basal body elimination, as well as the initiation of mucus production and secretion. Our work describes two modes for MCC loss during vertebrate development, the signaling regulation of these processes, and demonstrates that even cells with extreme differentiation features can undergo direct fate conversion.
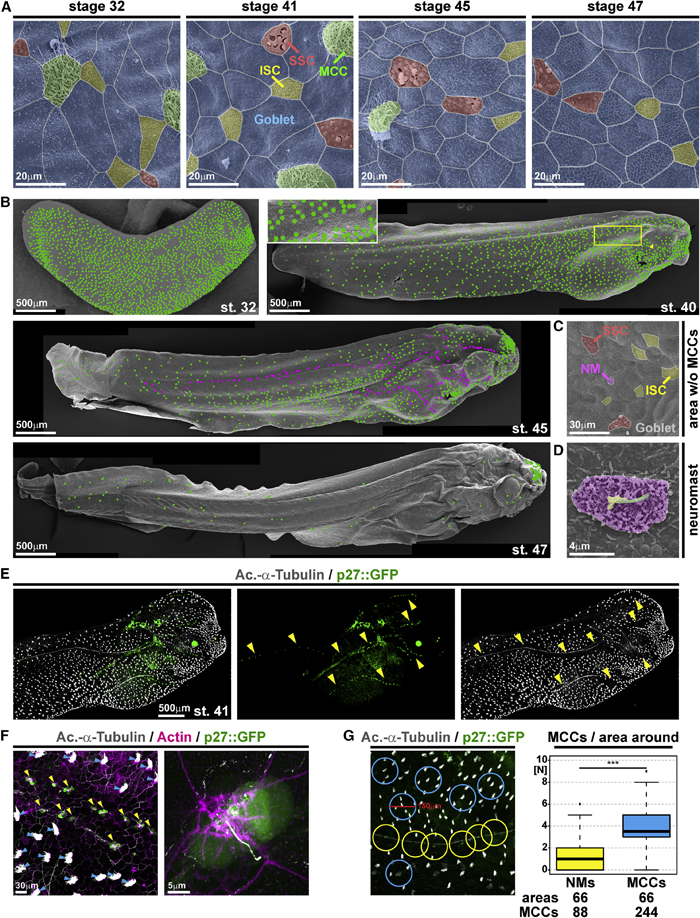
Figure 1. Characterization of epidermal MCC loss during Xenopus development
(A–D) Pseudo-colored scanning electron micrographs from developmental stages (st.) 32 through 47. Cf. Figure S1 for non-colored images.
(A) Analysis of the changing composition of mucociliary cell types in the epidermis shows progressive loss of MCCs (green), while ionocytes (ISCs, yellow), small secretory cells (SSCs, red), and mucus-secreting goblet cells (blue) remain present.
(B) Analysis of MCC-loss patterns on whole tadpoles. MCCs are marked by green dots. Neuromasts are marked by magenta dots. Box indicates a magnified area. Arrowhead indicates MCC loss ventral to the eye.
(C) Magnification of skin area devoid of MCCs at st. 45 reveals the presence of lateral line neuromast (NM, purple).
(D) Magnified image of a neuromast. Kinocilium (green), stereocilia (yellow), support cells (purple). (A–D) st. 32 n = 7; st. 40/41n = 6; st. 45n = 3; st. 47n = 3 embryos. (E) Confocal micrograph of NMs (p27::GFP, green; yellow arrowheads) and MCCs (Ac.-a-Tubulin, gray). The picture was reconstructed from multiple images. (F) Confocal micrograph of p27::GFP (green; NMs marked by yellow arrowheads) transgenic tadpole stained for cilia (Ac.-a-Tubulin, gray; MCCs marked by blue arrowheads) and F-actin (actin, magenta) shows lack of MCCs around NMs and GFP expression in a subset of ciliated neuromast cells. (E and F) n = 3 embryos. (G) Quantification of MCCs in areas with 150 mM diameter around NMs (yellow) or MCCs (blue) shows a reduced density of MCCs around NMs in the specimen depicted in (E). Mann Whitney test, ***p < 0.001. Images in (B), (E), and (F) were reconstructed from multiple individual micrographs.
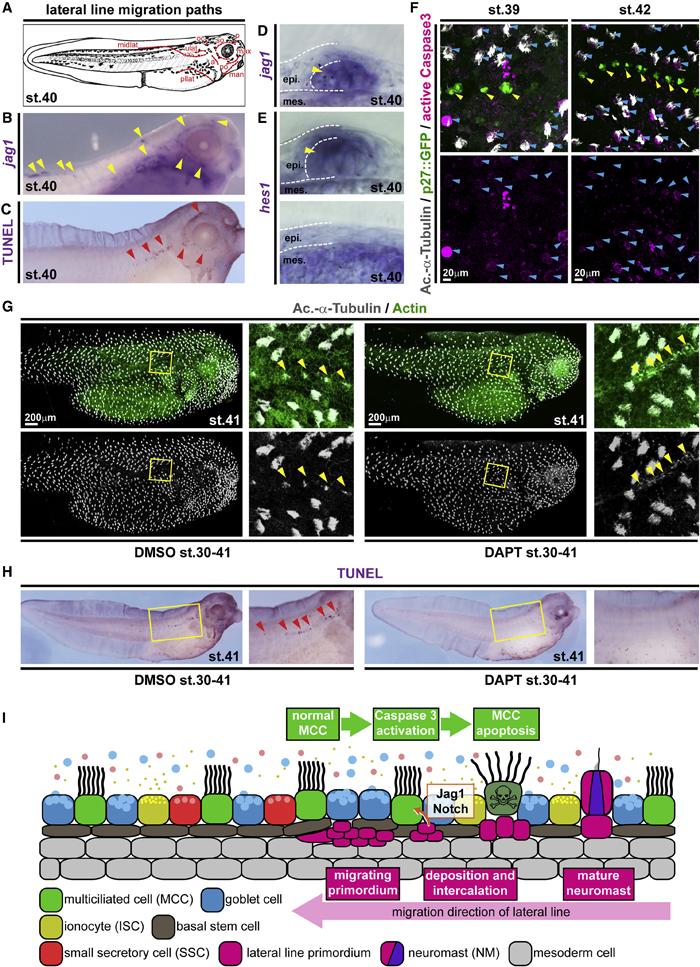
Figure 2. Local loss of MCCs is induced by Notch signaling from the emerging lateral line
(A) Schematic representation of lateral line migration patterns in st. 40 Xenopus tadpoles.
(B) In situ hybridization shows jagged1 (jag1, purple) expression in the lateral line primordium and in NMs (yellow arrowheads). n = 3 embryos. (C) TUNEL staining (purple) reveals apoptotic cells clustering along the lateral line migration paths (red arrowheads). n = 13 embryos.
(D and E) Sections of NMs and epidermal area after in situ hybridization staining for jag1 (D) and hes1 (E) at stage 40. (D) n = 4 embryos. (E) n = 4 embryos. (F) Active Caspase 3 (magenta) staining in MCCs (Ac.-a-Tubulin, gray; blue arrowheads) located in proximity to NMs (p27::GFP, green; yellow arrowheads) at stages 39 (n = 6 embryos) and 42 (n = 5 embryos).
(G) Notch signaling inhibition by DAPT prevents lateral line-induced MCC (Ac.-a-Tubulin, gray) loss in the presence of neuromasts (yellow arrowheads). F-actin (actin, green) was used as a counterstain in confocal images. (DMSO n = 11; DAPT n = 13 embryos)
(H) Notch signaling inhibition by DAPT application prevents induction of apoptosis (TUNEL staining, purple; red arrowheads) by the lateral line. Magnified areas in (G and H) are indicated by yellow boxes. Cf. Figure S3 for quantification of results. (DMSO n = 24; DAPT n= 25 embryos) Images in (F) and (G) were reconstructed from multiple individual micrographs.
(I) Schematic representation of lateral line-induced MCC removal.
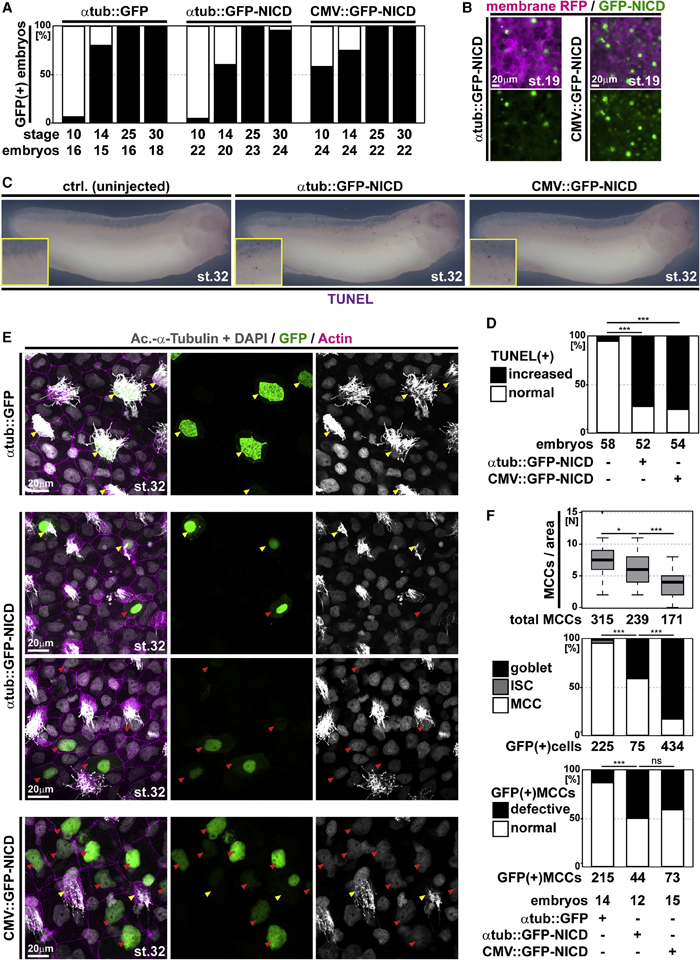
Figure 4. Ectopic Notch signaling induces apoptosis and cell fate change in MCCs
(A) Analysis of GFP expression in embryos injected with a control construct (atub::GFP) or GFP-NICD constructs under control of a MCC-specific promoter (atub::GFP-NICD) or a ubiquitous promoter (CMV::GFP-NICD) show different onset of promoter activation. Graph depicts GFP fluorescence in % of analyzed embryos. n embryos per stage and construct analyzed are indicted below the graph.
Adapted with permission from Cell Press on behalf of Developmental Cell: Tasca et al. (2021). Notch signaling induces either apoptosis or cell fate change in multiciliated cells during mucociliary tissue remodeling. Dev Cell. 2021 Jan 4. DOI:https://doi.org/10.1016/j.devcel.2020.12.005
