Green oxygen power plants in the brain rescue neuronal activity
Suzan Özugur, Myra N Chávez, Rosario Sanchez-Gonzalez, Lars Kunz, Jörg Nickelsen, Hans Straka
iScience. 2021 Oct 13;24(10):103158. doi: 10.1016/j.isci.2021.103158. eCollection 2021 Oct 22.
Click here to view article at iScience.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
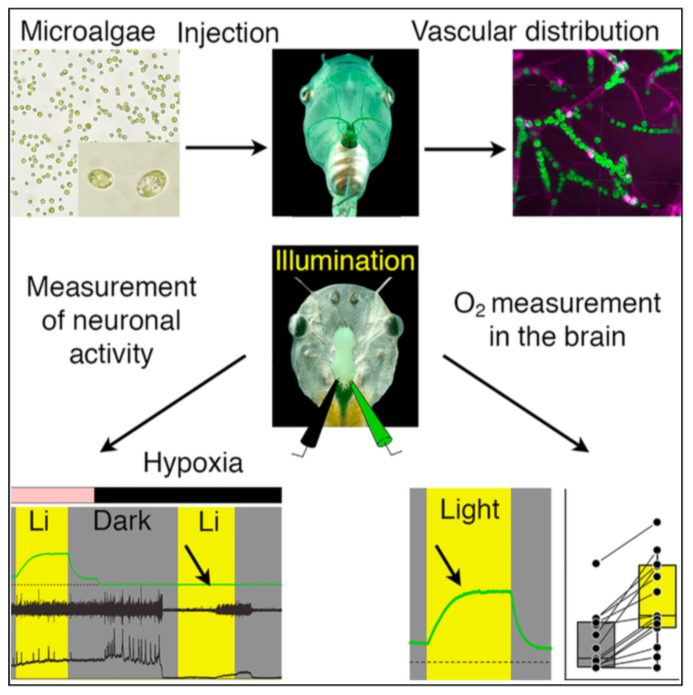
Highlights
• Transcardially injected microalgae accumulate in brain blood vessels of Xenopus
• Upon illumination, green algae and cyanobacteria robustly produce O2 in the brain
• After hypoxic loss of brain function, photosynthetic oxygen rescues neural activity
Abstract
Neuronal activity in the brain depends on mostly aerobic generation of energy equivalents and thus on a constant O2 supply. Oxygenation of the vertebrate brain has been optimized during evolution by species-specific uptake and transport of O2 that originally derives from the phototrophic activity of prokaryotic and eukaryotic organisms in the environment. Here, we employed a concept that exploits transcardial injection and vascular distribution of unicellular green algae or cyanobacteria in the brain of Xenopus laevis tadpoles. Using oxygen measurements in the brain ventricle, we found that these microorganisms robustly produce sizable amounts of O2 upon illumination. In a severe hypoxic environment, when neuronal activity has completely ceased, the photosynthetic O2 reliably provoked a restart and rescue of neuronal activity. In the future, phototrophic microorganisms might provide a novel means to directly increase oxygen levels in the brain in a controlled manner under particular eco-physiological conditions or following pathological impairments.
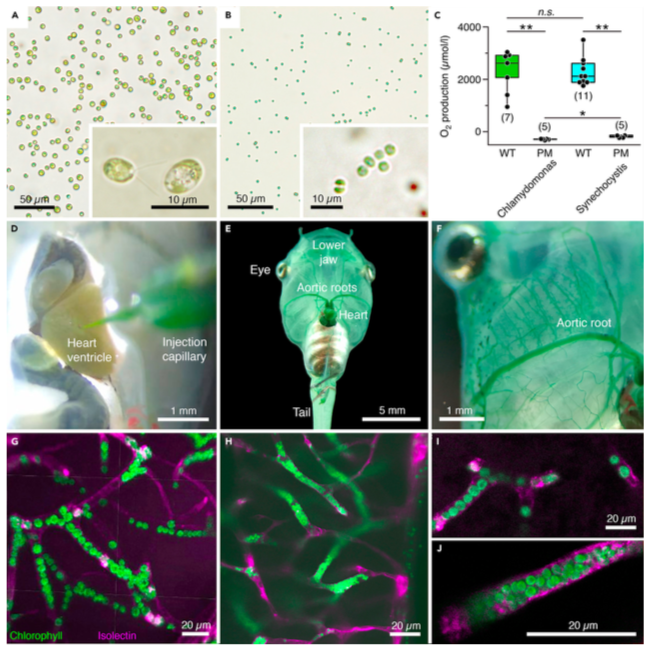
Figure 1. Transcardial injection and accumulation of photosynthetic microorganisms in blood vessels of Xenopus laevis tadpoles
(A and B) Microphotographs depicting C. reinhardtii (A) and Synechocystis 6803 (B) before injection; insets show the microorganisms at higher magnification. (C) Light-induced O2 production and lack thereof, respectively, in solutions (108 cells/mL) of wild-type (WT) and photomutant (PM) strains of the two species; number of experiments indicated in parentheses.
(D–F) Photographs depicting pressure-injection of a Synechocystis 6803 solution into the heart (D) and distribution of the microorganisms through the aortic roots (E,F) and smaller blood vessels (green staining) of the body (E) and lower jaw (F).
(G–J) Confocal reconstruction of hindbrain tissue, illustrating accumulated C. reinhardtii (G and I) and Synechocystis 6803 (H and J) inside blood vessels; microorganisms appear in green due to chlorophyll autofluorescence and blood vessels in magenta by isolectin-stained endothelial walls. Calibration bars represent 50 mm (A and B), 10 mm (insets), and 1 mm (D and F), 5 mm (E), 20 mm (G–J). *, p < 0.05; **, p < 0.01; Mann Whitney U-test, Bonferroni-corrected; n.s. not significant.
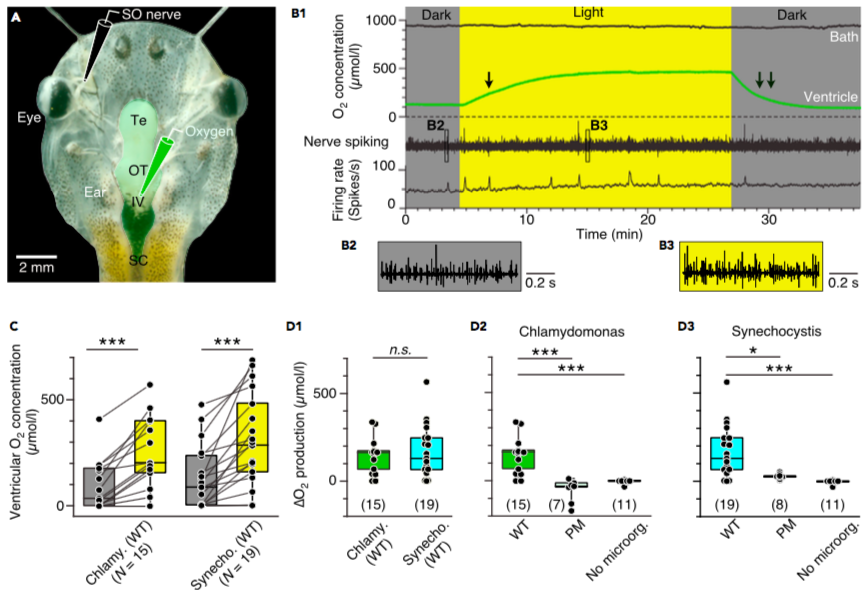
Figure 2. Photosynthetic oxygen production in the brain
(A) Semi-intact head preparation, illustrating the recording of O2 concentrations in the IVth ventricle (green electrode) and of superior oblique (SO) nerve spike discharge (black electrode).
(B) Episode (B1) of O2 concentration recording in the bath chamber (black trace) and IVth ventricle (green trace) and of concurrent multi-unit SO nerve spiking and mean firing rate (black trace) in darkness and light in a preparation containing C. reinhardtii; the ventricular O2 concentration increases during illumination with a slower rise (single arrow) and faster decay (double arrow); insets depict SO nerve spiking in darkness (B2) and light (B3) at higher temporal resolution.
(C) Boxplot depicting ventricular O2 concentrations in darkness (gray bars) and light (yellow bars) in preparations containing wild-types of C. reinhardtii or Synechocystis 6803; ***, p < 0.001, Wilcoxon signed-rank test.
(D) Boxplots depicting light-induced changes (D) of O2 concentrations in preparations containing either wild-type (WT) microorganisms (D1) in comparison with photomutant (PM) strains or controls without C. reinhardtii (D2) or Synechocystis 6803 (D3); number of experiments indicated in parentheses. *, p < 0.05, ***, p < 0.001, Mann Whitney U-test, Bonferroni-corrected; n.s. not significant.
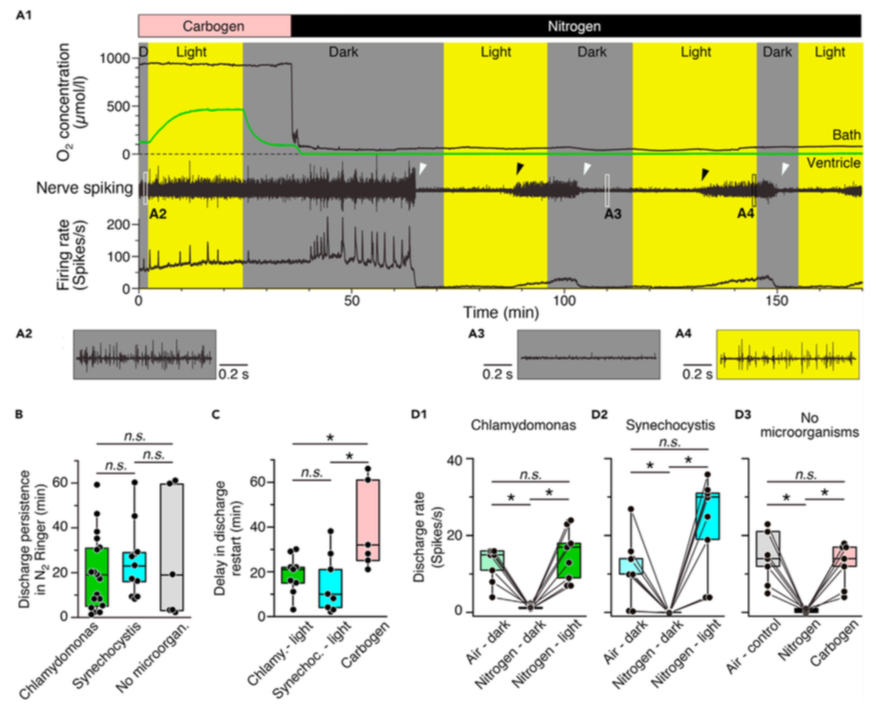
Figure 3. Photosynthetic oxygen production rescues neuronal activity under hypoxic conditions
(A) Episode (A1) of O2 concentration recording in the bath chamber (black trace) and IVth ventricle (green trace) and of concurrent superior oblique nerve spiking and mean firing rate (black trace) at hyperoxic (Carbogen) and hypoxic (Nitrogen) bath O2 levels during alternating periods of darkness and light in a preparation containing wild-type Synechocystis 6803; note that nerve spiking under hypoxic conditions reversibly ceases in darkness (white arrow heads) but can be restarted during illumination (black arrow heads); insets depict nerve spike discharge under control (A2) and hypoxic conditions in darkness (A3) and light (A4) at higher temporal resolution.
(B–D) Boxplots depicting the delay of discharge cessation under hypoxic conditions in darkness (B), light- or carbogen-induced restart of spiking (C) and magnitudes of re-established firing rates (D) with either C. reinhardtii (green bars) or Synechocystis 6803 (cyan bars) in the vascular system as compared to the firing rate following carbogen-induced restart of spiking (gray/pink bars) in darkness. *, p < 0.05, Mann Whitney U-test in (B) and (C), Bonferroni-corrected; Wilcoxon signed-rank test in (D), n.s. not significant.
Adapted with permission from Cell Press on behalf of iScience: Ozugur et al. (2021). Green oxygen power plants in the brain rescue neuronal activity. iScience. 2021 Oct 13;24(10):103158. doi: 10.1016/j.isci.2021.103158. eCollection 2021 Oct 22.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2021-12-20