Pleiotropic role of TRAF7 in skull-base meningiomas and congenital heart disease
Proc Natl Acad Sci U S A 2023 Apr 18;12016:e2214997120. doi: 10.1073/pnas.2214997120.
Mishra-Gorur K, Barak T, Kaulen LD, Henegariu O, Jin SC, Aguilera SM, Yalbir E, Goles G, Nishimura S, Miyagishima D, Djenoune L, Altinok S, Rai DK, Viviano S, Prendergast A, Zerillo C, Ozcan K, Baran B, Sencar L, Goc N, Yarman Y, Ercan-Sencicek AG, Bilguvar K, Lifton RP, Moliterno J, Louvi A, Yuan S, Deniz E, Brueckner M, Gunel M.
Click here to view article at PNAS.
Click here to view article on PubMed.
Click here to view article on Xenbase.
Significance
The genetic basis for the increased risk of cancer with congenital heart disease (CHD) is largely unclear. Mishra-Gorur et al. is significant because it identifies TRAF7 (Tumor necrosis factor receptor-associated factor 7) mutations in both, brain tumors (meningiomas) and CHD. While somatic mutations in TRAF7 underlie anterior skull-base meningiomas, here they report the inherited mutations of TRAF7 that cause CHD and show that the shared genetics of the two disparate pathologies can be traced to the common developmental origin of the two tissues from the neural crest. This work demonstrates that these mutations act as dominant negative by dimerizing with wild-type TRAF7 and disrupting its function and ascribe a unique role for TRAF7 in ciliogenesis and intraflagellar transport.
Abstract
While somatic variants of TRAF7 (Tumor necrosis factor receptor-associated factor 7) underlie anterior skull-base meningiomas, here we report the inherited mutations of TRAF7 that cause congenital heart defects. We show that TRAF7 mutants operate in a dominant manner, inhibiting protein function via heterodimerization with wild-type protein. Further, the shared genetics of the two disparate pathologies can be traced to the common origin of forebrain meninges and cardiac outflow tract from the TRAF7-expressing neural crest. Somatic and inherited mutations disrupt TRAF7-IFT57 interactions leading to cilia degradation. TRAF7-mutant meningioma primary cultures lack cilia, and TRAF7 knockdown causes cardiac, craniofacial, and ciliary defects in Xenopus and zebrafish, suggesting a mechanistic convergence for TRAF7-driven meningiomas and developmental heart defects.
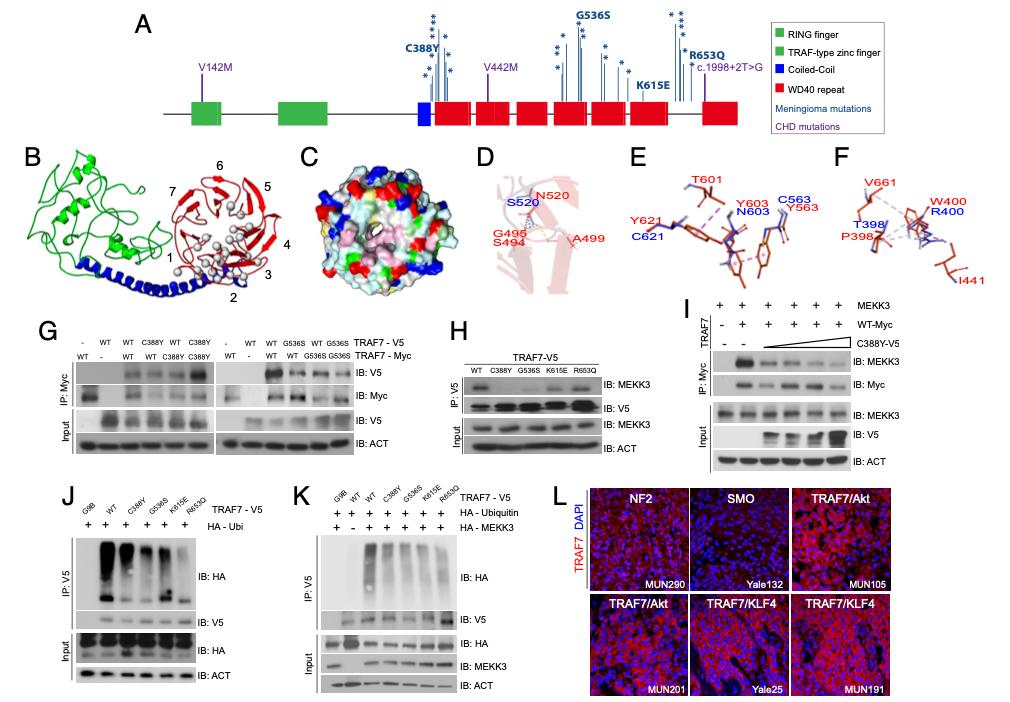
Fig. 1. TRAF7 mutations disrupt protein structure and interactions. (A) Protein structure of TRAF7; developmental variants V142M, V442M, and c.1998+2T>G and the locations of previously reported (16) meningioma-associated mutations (asterisks; 93% map to the WD40 domains), including those analyzed in this study. (B) Predicted structure of TRAF7. The RING finger (green), coiled-coil (blue), and putative 7-WD40-repeat-containing putative ligand-binding (red) domains are indicated. The mutations (gray circles) are primarily localized to one face of the latter. (C) Representation of the WD40-domain molecular surface, exhibiting a hydrophobic patch (pink, reflecting concentration of white [small hydrophobic] and magenta [aromatic] residues) surrounding the pore of the β-propeller surface. Yellow: cysteine; pale green: proline; green: glycine; cyan: polar; blue: positively charged residues; red: negatively charged residues. (D) The N (red) to S (blue) mutation at position 520 changes hydrogen bonding (yellow dashes) between the β-strands of the preceding blade of the WD40 β-propeller domain. (E) Substitution of Y residues 563, 603, and 621 (red) with charged and polar (blue) residues results in loss of hydrophobic interaction (purple – light pink dashes). (F) The W (red) to R (blue) substitution at position 400 abrogates hydrophobic interactions with several residues involving multiple β-propeller units. (G) WT and meningioma-associated mutant forms of TRAF7 can form homo- and hetero-dimers. (H) Mutant TRAF7 disrupts the interaction with endogenous MEKK3. Coimmunoprecipitation analysis in HEK293 cells. (I) Low concentrations of mutant TRAF7 (C388Y) are sufficient to disrupt the interaction of WT TRAF7 with MEKK3. Plasmids expressing C388Y (1, 2, 3, or 4 μg) and WT (4 μg) TRAF7 were cotransfected in HEK293 cells followed by immunoprecipitation for WT-TRAF7. (J and K) TRAF7 mutants display reduced ubiquitination in the absence (J) or presence (K) of exogenous MEKK3. (L) Surgically resected TRAF7-mutant meningiomas highly express TRAF7 (genotypes of tumors shown: MUN290: NF2; Yale 132: SMO W535L; MUN105: TRAF7 R641C/AKT1 E49K; MUN201: TRAF7 L580del/AKT1 E49K; Yale 25: TRAF7 G536S/KLF4 K409Q; MUN191: TRAF7 K615E/KLF4 K409Q). (Scale bar, 50 μm.) Confocal images captured under identical settings.
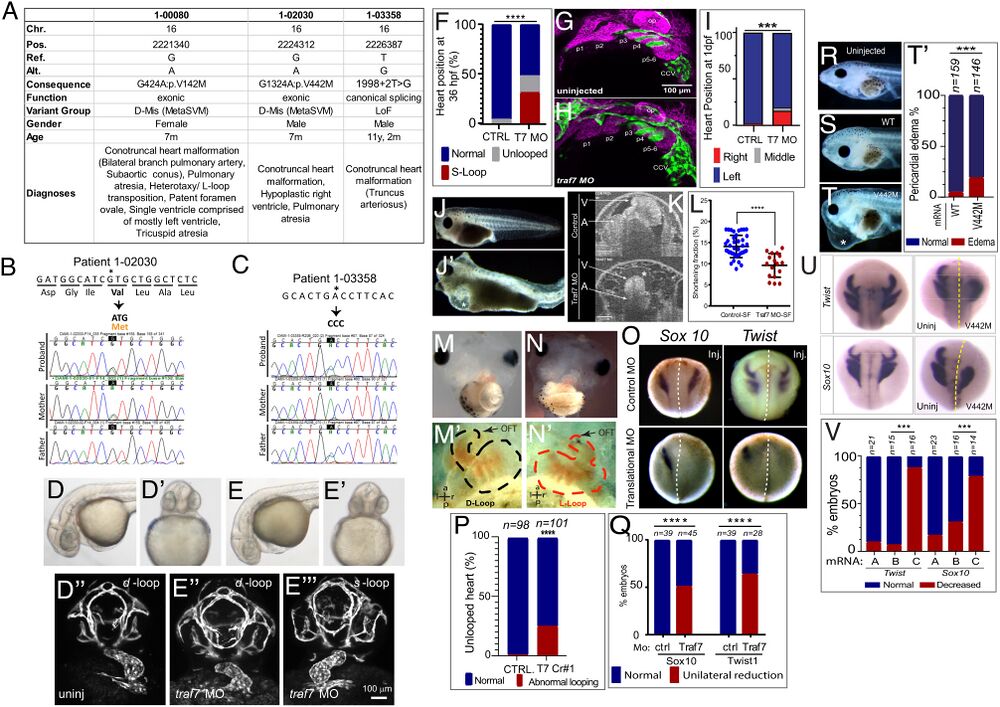
Figure 2. TRAF7 mutations cause congenital heart defects: reduction of TRAF7 in zebrafish (D–H) and Xenopus tropicalis (I–P) as well as overexpression of mutant TRAF7 in X. tropicalis causes developmental defects (Q–U). (A) Clinical manifestations of patients harboring inherited TRAF7 heterozygous developmental mutations. (B and C). Sanger sequencing traces of patients 1-02030 and 1-03358 and their clinically unaffected parents. The mutations are indicated in bold, and asterisk marks the mutated residue. (D–F) Injection of control (D–D’’) or splice-site (E–E’’’) TRAF7 MO in tg(kdrl:GFP;gata-1:dsRed) embryos results in pronounced heart looping defects at 36 hpf. (F). Quantification of embryos displaying cardiac looping defect ****: P < 0.0001 (Fisher’s exact test; n= # of embryos). (G and H). Traf7 morphants exhibit reduced sox10 expression, disorganized pharyngeal arches. (H) Uninjected zebrafish (Tg:kdr:GFP, sox10:mRFP) embryo at 30 hpf showing pharyngeal arches 1 to 6 (p1 to p6). The otic placode (op) and common cardinal vein (CCV) are also labeled. (I) TRAF7 morphant at the same stage. Note the reduced size of the pharyngeal arches, disorganization of p3 to p6, and reduced Sox10 expression. Green channel: endothelial cells; magenta: Sox10-expressing cells. (I) Distribution of heart position in control and TRAF7 1-d postfertilization morphants. Control: Left = 97.29% ± 3.29; middle= 0.83% ± 1.67; right= 1.88 ± 2.19, from 112 embryos total. TRAF7: Left = 76.31% ± 9.32; Middle = 3.07% ± 5.10; Right = 20.62 ± 9.99, from 112 embryos total. Two-way ANOVA with Bonferroni’s multiple-comparison test, P = 0.0004 for left heart position. J−K. Injection of control (J) or splice-site (J’) TRAF7 MO in one-cell stage Xenopus embryos results in extensive pericardial edema (asterisk). Optical coherence tomography (OCT) highlights the edema (asterisk) and malformed heart (K), reflected in a significant reduction of the shortening fraction (L) at 3 dpf (stage 46) (Movie S3). Mann–Whitney test (scatter plot mean ± SD; n = 17, TRAF7 splice-site MO injected; n = 41, control MO injected.) M–N’ TRAF7 CRISPR (CR#1) injection in one-cell stage Xenopus embryos results in pronounced heart looping defects at 48 hpf (N, N’) as compared to controls (M, M’) (Movie S4). OFT: outflow tract, V: ventricle. O. In situ hybridization analysis of Xenopus embryos (stage 16 to 18, 15 hpf): Unilateral injection (Inj.) with splice-site TRAF7, MO at two-cell stage shows disrupted expression of neural crest markers Sox10 and Twist on the injected side when compared to the internal uninjected control. (P) Quantification of embryos displaying cardiac looping ****: P < 0.0001 (Fisher’s exact test; n= # of embryos). ( Q) Quantification of control or TRAF7 splice-site MO-injected embryos analyzed for Sox10 and Twist expression. n = number of embryos. ****: P < 0.0001 (Fisher’s exact test; n = # of embryos). (R–T’) Severe pericardial edema in Xenopus embryos following injection of TRAF7 mRNA encoding mutant form V442M (T) but not WT (S) as compared to uninjected embryos (R). Quantification of embryos displaying pericardial edema (T’), ***: P < 0.001 (Fisher's exact test, n = # of embryos). (U) In situ hybridization analysis of Xenopus embryos (stage 17, 18 hpf): Decreased expression of the neural crest markers Twist and Sox10 following unilateral injection of TRAF7 V442M mRNA at the 2-cell-stage. (V). Quantification of control or TRAF7 splice-site MO-injected embryos analyzed for Sox10 and Twist expression. A = Control, B = WT TRAF7, C = V442M TRAF7. ***:P < 0.001: Pairwise Fisher’s exact test with FDR correction, n = # of embryos.
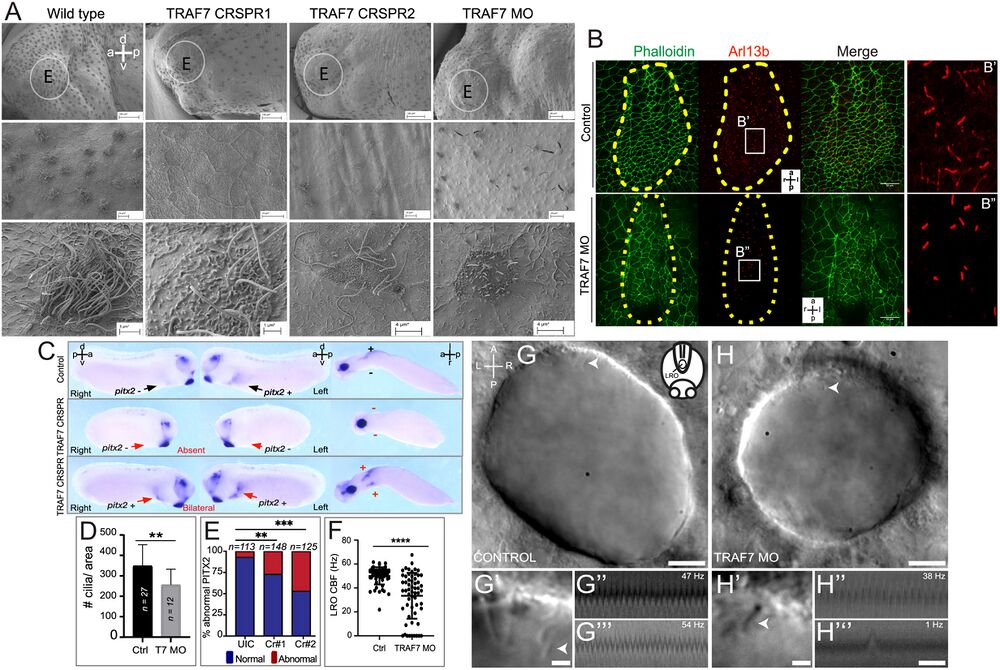
Figure 5. TRAF7 knockdown in Xenopus tropicalis (A–G) and zebrafish (H–J’’’) affects mono- and multi-cilia in the left–right organizer (LRO). (A) Scanning electron microscopic images of Xenopus epidermis reveal defective cilia formation on TRAF7 depletion with either CRISPR #1, #2, or MO. Circled areas are magnified in the bottom panels. (Scale bars: Top row: 100 µm, middle row: 10 µm, bottom row: 3 µm.) (B) Xenopus embryos were injected at 1-cell stage and dorsal explants were prepared at stage 17 to visualize the left–right organizer (LRO). Specimens were processed for immunohistochemistry (IHC) to assess ciliation rate and cell surface area. Compared to uninjected controls, TRAF7 morphants have fewer cilia, as shown by acetylated tubulin (red) and phalloidin (actin, to outline cell boundaries; green). a = anterior, l = left, p = posterior, r = right. C. Analysis of pitx2c expression in stage 28 to 30 Xenopus embryos. Embryos are viewed laterally from the right (first column), the left (second column), and ventrally (third column). Expression of pitx2c is normally in the left lateral plate mesoderm (LPM, black arrow). CRISPR-mediated TRAF7 knockdown results in abnormal absent pitx2c expression with no pitx2c mRNA found in the left or right LPM (Middle panel, red arrows); or abnormal bilateral pitx2c expression with pitx2c mRNA found in both left and right LPM (Bottom panel, red arrows). (D) Bar plot demonstrating quantification of ciliation in relation to cell surface area in the LRO of TRAF7 morphant and control X. tropicalis embryos in (C). **: P < 0.00: t test with Welch correction, n= # of embryos. E. Quantification of pitx2c expression in uninjected controls (UICs) and traf7-G0 mutants by sgRNA#1 and #2. Abnormal includes absent and bilateral pitx2c expression. Statistical calculations were performed using a Chi-square test comparing the number of affected embryos against the number of wild-type embryos. **P < 0.01, ***P < 0.001; n, number of analyzed tadpoles. (F–H). TRAF7 is required for proper motility of cilia in zebrafish LRO. (F) Quantification of the ciliary beating frequency (CBF) in control (60 cilia from eight embryos) and TRAF7 morphants (58 cilia from eight embryos). Mean control CBF= 50.5 Hz ± 7.14. Mean TRAF7 CBF = 33.47 ± 19.15. Two-sample t tests, P = 6.5 × 10−9; mean ± SD. Representative images of the LRO cilia of a control MO-injected embryo (eight somite stage) (G and G’) and of a TRAF7 MO-injected embryo (six somite stage) (H and H’) (Movies S7 and S8). (Scale bars: G, H = 10 µm; G’, H’ = 2 µm.) White arrows indicate cilia. G’ and H’ are close-ups of the imaged regions in the anterior side of the LRO in G and H (Movies S9–S12). Representative kymographs of two individual “control” cilia (G” and G’’’) and of TRAF7 knockdown cilia (H’’ and H’’’). Kymograph total duration 500 ms. (Scale bar, 100 ms.) Movie S6.
Adapted with permission from the Proceedings of the National Academy of Sciences: Mishra-Gorur et al. (2023). Pleiotropic role of TRAF7 in skull-base meningiomas and congenital heart disease. Proc Natl Acad Sci U S A 2023 Apr 18;12016:e2214997120. doi: 10.1073/pnas.2214997120.
