Competence for neural crest induction is controlled by hydrostatic pressure through Yap
Delan N. Alasaadi, Lucas Alvizi, Jonas Hartmann, Namid Stillman, Prachiti Moghe, Takashi Hiiragi & Roberto Mayor
Nat Cell Biol. 2024 Mar 18. doi: 10.1038/s41556-024-01378-y.
Click here to view article at Nature Cell Biology.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Abstract
Embryonic induction is a key mechanism in development that corresponds to an interaction between a signalling and a responding tissue, causing a change in the direction of differentiation by the responding tissue. Considerable progress has been achieved in identifying inductive signals, yet how tissues control their responsiveness to these signals, known as competence, remains poorly understood. While the role of molecular signals in competence has been studied, how tissue mechanics influence competence remains unexplored. Here we investigate the role of hydrostatic pressure in controlling competence in neural crest cells, an embryonic cell population. We show that neural crest competence decreases concomitantly with an increase in the hydrostatic pressure of the blastocoel, an embryonic cavity in contact with the prospective neural crest. By manipulating hydrostatic pressure in vivo, we show that this increase leads to the inhibition of Yap signalling and impairs Wnt activation in the responding tissue, which would be required for neural crest induction. We further show that hydrostatic pressure controls neural crest induction in amphibian and mouse embryos and in human cells, suggesting a conserved mechanism across vertebrates. Our work sets out how tissue mechanics can interplay with signalling pathways to regulate embryonic competence.
Fig. 1: Loss of neural crest competence correlates with increased hydrostatic pressure. a, Schematic of neural crest ectopic induction assay using DLMZ as the inducer (grey), grafted into host blastocoel cavity (red). b, In situ hybridization analysis of foxd3 and snai2 at stage (St) 17 and 18, respectively, seen in ventral view and dorsal view as inset. c, Spread of data indicating the percentage of embryos with ectopic induction analysed with different neural crest (NC) markers. d, Quantification of neural crest competence at the indicated stages; 10, 11 and 12 normalized to control with no graft. Embryos that exhibited ectopic induction are represented as Ectopic+ (red), and embryos with no ectopic induction are shown as Ectopic (black). e, Micro-CT of a whole mount embryo (grey) at stages 10, 11 and 12 showing blastocoel cavity (red). f,g, Quantification of blastocoel volume (f) and hydrostatic pressure (g) at stages 1012. Scale bars, 450um for ventral and 200um for dorsal (b) and 300um (e). Statistical analysis was performed using two-sided Dunnetts tests (*P=0.0164 (g), ****P0.0001 (d,f), 95% CI). Data are mean and s.d. (c). Box plots (f,g) show median, 25th and 75th percentiles, and whiskers extending to minimum and maximum values. Three independent experiments (c,d). n=17 st10, 19 st11 and 12 st12 embryos (f) and n=19 st10, 13 st11, and 22 st12 embryos (g).
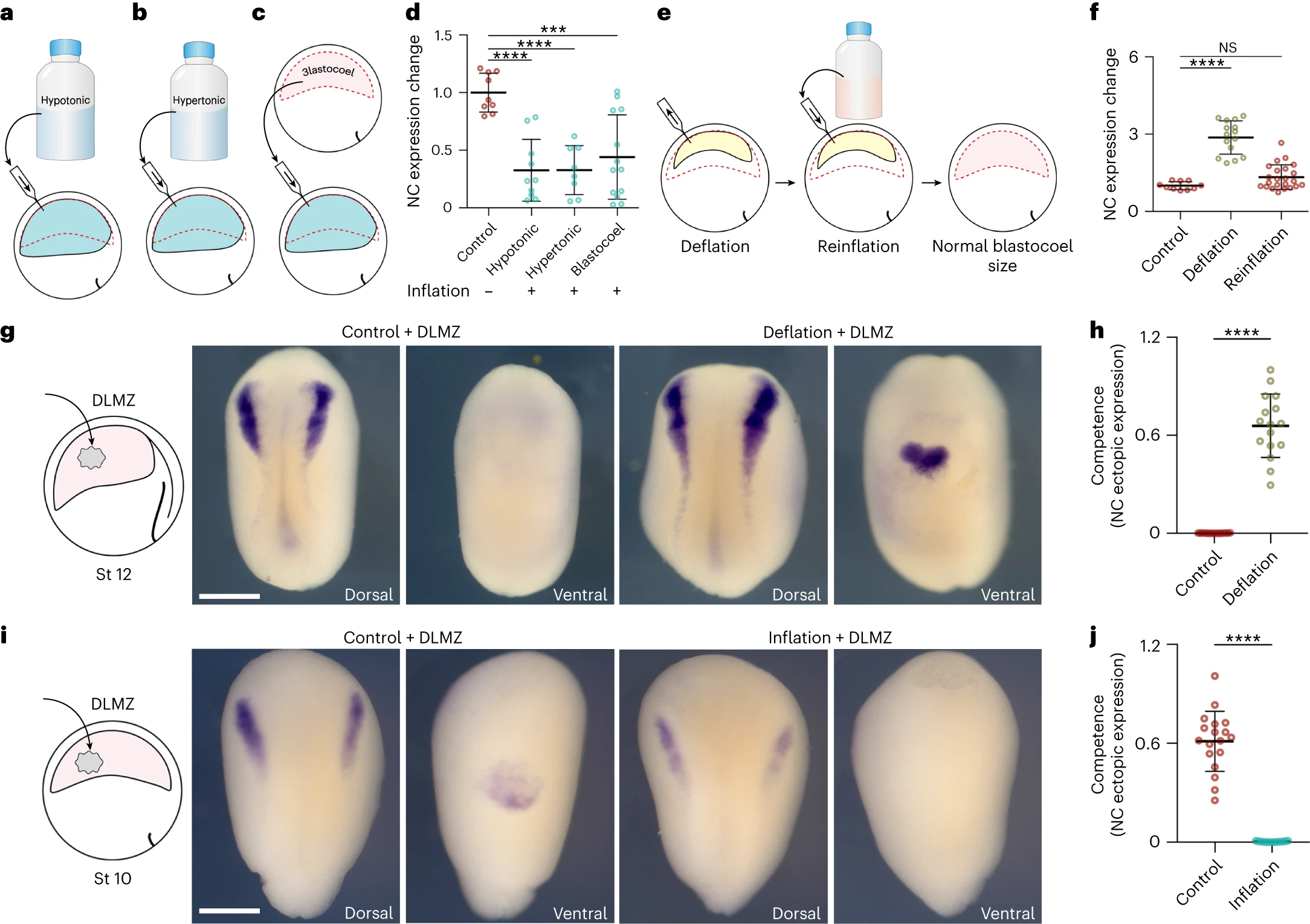
Fig. 3: Hydrostatic pressure controls neural crest competence. ac, Schematic of inflating embryo with hypotonic (a), hypertonic (b) and blastocoel fluid (c). d, Spread of data points comparing the change in snai2 expression at stage 14. e, Schematic of reinflating an embryo to normal blastocoel size after deflation with a saline solution. f, Spread of data points comparing changes in snai2 expression at stage 14. g,i, Left to right: the schematic of DLMZ graft assay into stage 12 and 10 embryos, respectively and analysed via ISH for snai2 at stages 18 and 16, respectively. h,j, Quantification of neural crest competence assay during deflation (h) and inflation (j) of Xenopus embryos. Scale bar, 450um (g,i). Data are mean and s.d. Statistical analysis was performed using two-sided Dunns test and unpaired t-tests (NS, P=0.2590 (f), ***P=0.0001 (d), ****P0.0001 (d,f,h,j), 95% CI). n=9 control, 10 hypotonic, 8 hypertonic and 13 blastocoel embryos (d), n=10 control, 1 deflation and 23 reinflation embryos (f), n competence=16 control and deflation embryos (h), n competence=18 control and inflation embryos (j).
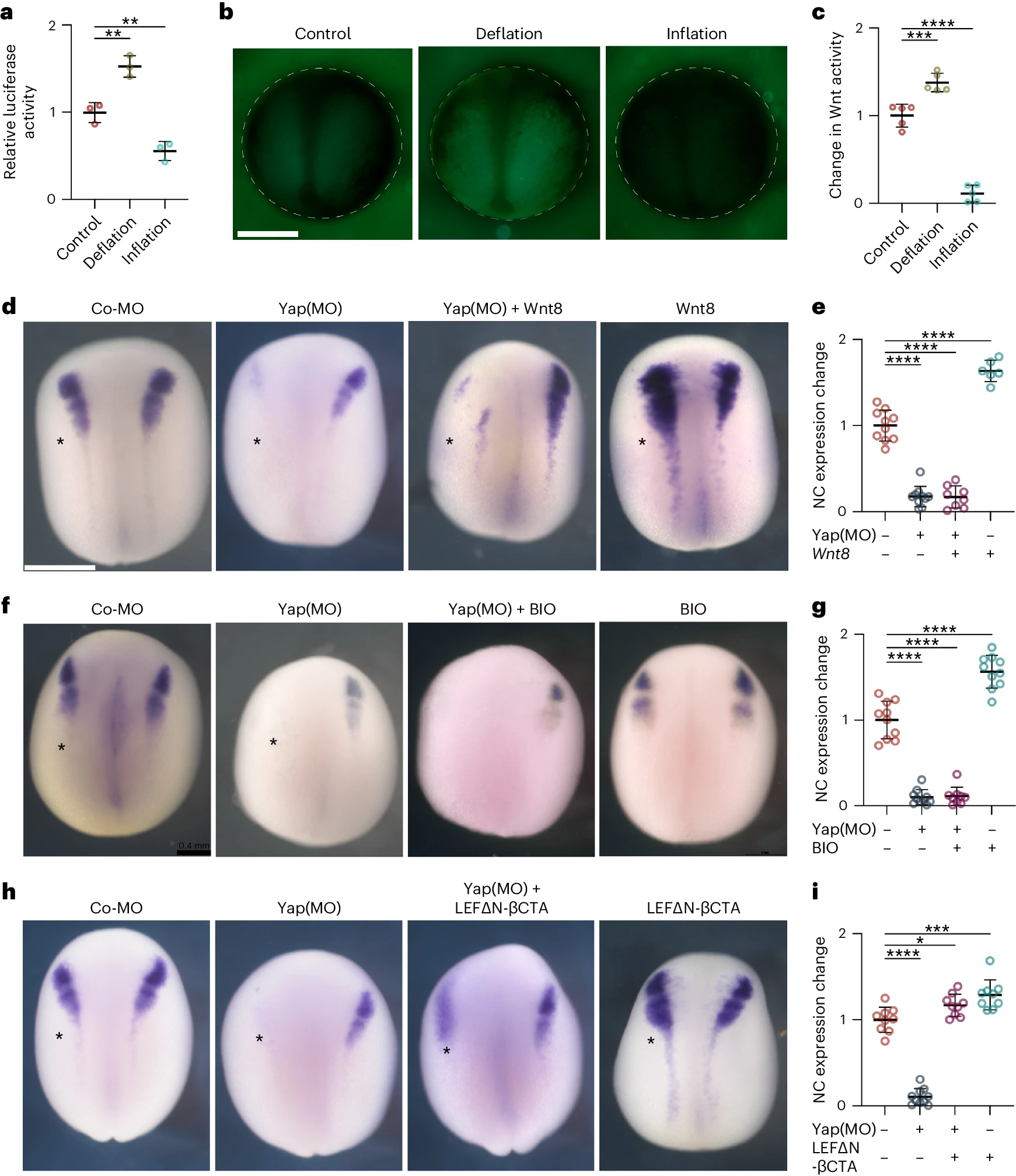
Fig. 4: Hydrostatic pressure regulates Yap activity. a, Relative luciferase activity of super Top flash after the indicated treatments, normalized to Fop flash. b, Xenopus transgenic embryos Tg(pbin7Lef-dGFP) to detect Wnt activity at stage 12.5 after the indicated treatments. c, Quantification of GFP intensity normalized to control embryos. d,f,h, In situ hybridization of snai2 at stages 16 and 17, after the indicated injections at the eight-cell stage. Asterisks indicate the injected side. Embryos injected with control Yap morpholino (Co-MO), Yap morpholino (Yap(MO)), or Wnt pathway activators. e,g,i, Quantification of snai2 expression level analysed via ISH during inhibition of Yap, and with Wnt8 mRNA (e), BIO (g), or an active form of -catenin (LEFN-CTA; i). Scale bars, 450m. Data are mean and s.d. Three independent experiments; each point represents three replicates (a). n=5 embryos for each condition (c). Statistical analysis was performed using two-sided unpaired Dunnetts tests (*P=0.0378 (i), **P0.0062 (a), ***P=0.0003 (c,i), ****P0.0001 (c,e,g,i), 95% CI). n=10control, 10Yap-MO, 8Yap-MO + Xwnt8, 6Xwnt8 embryos (e). n=10control, 10Yap-MO, 9Yap-MO + BIO, 10BIO embryos (g). n=9control, 10Yap-MO, 9Yap-MO + -cat, 9-cat embryos (l).
Adapted with permission from Springer Nature on behalf of Nature Cell Biology: Alasaadi et al. (2024). Competence for neural crest induction is controlled by hydrostatic pressure through Yap. Nat Cell Biol. 2024 Mar 18. doi: 10.1038/s41556-024-01378-y.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2024-04-30