
|
Fig 1. In situ-hybridization localize transcripts of SmILK, SmPINCH and SmNck2 in the gonads of S. mansoni.Representative sections (5 μm) of schistosome couples are shown (males and females are indicated by biological symbols), which were hybridized with DIG-labeled antisense-RNA probes of SmILK (A–C), SmPINCH (G–I), SmNck2 (M-O). mRNA transcripts of SmILK were detected in the testes (t; see A), in mature (mo) and immature (io;) oocytes within the ovary (see B), the gastrodermis (g; see C), the parenchyma (p; see A-C) and the vitellarium (v; see C). Similar patterns were found for SmPINCH (testes, G; ovary, H; vitellarium and parenchyma, I) and SmNck2 (testes, M; ovary N; vitellarium, O). For control, sense-RNA probes were used for hybridization (SmILK, D-F: showing testes, ovary, vitellarium, gastrodermis and parenchyma, respectively; SmPINCH, J-L: showing testes, ovary, vitellarium, gastrodermis and parenchyma, respectively; SmNck2, P-R: showing testes, ovary, vitellarium, gastrodermis and parenchyma, respectively). Scale bars are 20 μm as indicated.
|

|
Fig 2. Co-Immunoprecipitation confirms complex formation.Co-Immunoprecipitation of HA-tagged Smβ-Int1 and V5-tagged SmILK (about 56 kDa), SmPINCH (about 42 kDa), and SmVKR1 (about 150 kDa) expressed in Xenopus oocytes. Anti-HA antibodies immunoprecipitated Smβ-Int1 together with SmILK, SmPINCH, and SmVKR1 upon co-expression in oocytes only when their wildtype forms were used (lane 11). Using deletion variants (DEL) of SmILK or SmPINCH, no immunoprecipitated interaction partners were detected (lanes 12, 13). No immunoprecipitated molecules were detected in further controls including individual molecules (lanes 1–6) or combinations with three of the four potential binding partners (lanes 7–10).
|
|
Fig 3. Complex formation occurs with the constitutively active SmVKR1 variant but not with a dead-kinase mutant.Co-Immunoprecipitation of HA-tagged Smβ-Int1 (β-Int-HA) and a selection (see Fig 2; the same numbering was used, except lanes 14, 15) of combinations of V5-tagged SmILK (ILK-V5), SmPINCH (PINCH-V5), and SmVKR1 (VKR1-V5) constructs in Xenopus oocytes. Anti-HA antibodies immunoprecipitated Smβ-Int1 together with SmILK, SmPINCH, endogenous Nck2, and SmVKR1 upon co-expression in oocytes only when their wildtype forms (lane 11) or the constitutively active form of SmVKR1XE (lane 14) were used. In these cases Xenopus Nck2 was part of the complex formation as detected in lanes 11 and 14 by anti-human Nck2 antibody. Using deletion variants (DEL) of SmILK (lane 12) and SmPINCH (lane 13), SmILK individually (lane 1), a combination of wildtype SmILK, SmPINCH and Smβ-Int1 (lane 7), or a dead kinase variant (KO) of SmVKR1 (lane 15), no immunoprecipitated interaction-partners were detected.
|

|
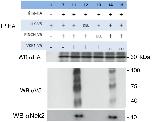
Fig 4. SmNck2 is part of the complex, and SmVKR1-phosphorylation occurs only when all complex-members are expressed.Western blot analysis of complex members expressed in Xenopus oocytes. (A) following transfection with SmVKR1, Smβ-Int1, SmILK, SmPINCH, and SmNck2 constructs (see below) coimmunoprecipitation with anti-HA and subsequent Western blot (WB) analyses with anti-HA (HA-tagged: Smβ-Int1) and anti-Flag (SmNck2ΔSH3) confirmed the presence of Nck2 in the complex. (B) following coimmunoprecipitation with anti-V5, Western blot (WB) analyses with anti-V5 (V5-tagged SmVKR1) and anti-PY20 confirmed tyrosine phosphorylation of SmVKR1 only in case of a combination with Smβ-Int1, SmILK, SmPINCH as well as SmNck2 and without ligand (L-Arg) addition (lane 1, WB PY20). When deletion mutants of SmILK (SmILKΔAnk1; lane 2), SmPINCH (SmPINCHΔLIM1, SmPINCHΔLIM4; lanes 3, 4), SmNck2 (SmNck2ΔSH3; lane 5), SmVKR1 (dk, dead kinase mutant; [22]; lane 6), or the ILK inhibitor QLT-0267 (1 μM; lane 7) were used, no tyrosine phosphorylation of SmVKR1 was detected. As a positive control, L-Arg was added as ligand, which induced SmVKR1 tyrosine phosphorylation as expected (lane 8).
|

|
Fig 5. Complex formation induces ERK2, JNK and AKT pathways in Xenopus oocytes in a ligand-independent way.Western blot analyses of signaling pathways triggered by Smβ-Int1 complex-mediated SmVKR1 activation in Xenopus oocytes. The schistosome complex members were expressed in Xenopus oocytes for 5 h with or without ligand. Oocyte lysates were analyzed to investigate the phosphorylation state of ERK2, JNK and AKT following SmVKR1- Smβ-Int1 complex formation. In the absence of L-Arg as ligand, and in case of the combination of Smβ-Int1, SmILK, SmPINCH, SmNck2, and SmVKR1 (lane 1) phosphorylation of ERK2 (ERK2P), JNK (JNKP), and AKT (AKTP) was observed. In cases of using mutated forms (SmILKΔAnk1, lane 2; SmPINCHΔLIM1, lane 3; SmPINCHΔLIM4, lane 4; SmNck2ΔSH3, laneΔ 5; SmVKR1 KO, lane 6) or the ILK inhibitor QLT-0267 (1 μM; lane 7), no phosphorylation of ERK2, JNK, and AKT was detected. As expected, phosphorylation of ERK2, JNK, and AKT was observed in SmVKR1-expressing oocytes stimulated by L-Arg (1 μM), which served as positive control (lane 8).
|
|
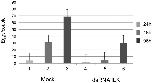
Fig 6. Egg production of worm couples decreases following RNAi suppressing SmILK activity in vitro.Compared to a MOCK control, egg production of couples treated with SmILK dsRNA significantly decreased from 48 h post treatment on.
|
|
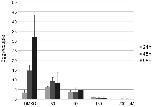
Fig 7. Egg production of worm couples decreases following QLT-0276 treatment in vitro.Analyses of egg production of adult S. mansoni in vitro following inhibitor treatment with the ILK inhibitor QLT-0276, which was used in different concentrations as indicated. Egg production significantly decreased from 48 h post treatment on in a concentration-dependent manner.
|
|
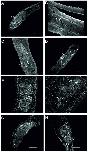
Fig 8. Morphological analyses show corresponding effects of QLT-0276 and RNAi in the ovary of female worms.CLSM analyses of paired females ((A) DMSO-treated female as control) under the influence of the inhibitor QLT-0276 (treatment for 3 days (B-D) or 14 days (E, F)) or ILK ds-RNA (25 μg, 4 days (G, H)) with a focus on the ovary. The ovary is a bulb-like structure with a smaller anterior part containing immature oogonia (iO) and a bigger posterior part containing mature primary oocytes (mO). As shown before [14, 15] the structure of the ovary and the integrity of oocytes were not negatively influenced by DMSO ((A) 3-day treatment). However, following treatment with 50 μM (B), 100 μM (C) or 200 μM (D) QLT-0276, mO appeared within the anterior part of the ovary (B, C) and iO in its posterior part (C, stars). Additionally, signs of oocyte degradation were observed in both parts of the ovary (arrows). After 14 days of in vitro culture the structure of the ovary of DMSO-treated females was unchanged (E) whereas ovaries of females treated with 10 μM QLT-0276 showed the deleterious phenotype (F) described above. In contrast to a control using GFP-dsRNA (G), in ILK dsRNA-treated paired females, the number of mO was smaller, they also appeared within the anterior part of the ovary (H), and iO in its posterior part, and signs of degeneration were observed, too (H). Scale bars are 75 μm (A, B, D), 50 μm (C, E), 45 μm (H) or 25 μm (E, F).
|
|
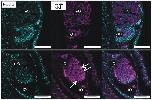
Fig 9. QLT-0276 reduces oocyte numbers and affects their morphology in paired females.Fluorescence microscopy of paired females with a focus on their ovaries. Females were treated for 3 days with DMSO (upper panel) or QLT-0276 (100 μM, lower panel) before staining with Hoechst 33342 (left) or anti-β-tubulin (middle). Merged images are shown right. Arrows indicate compact and rounded primary oocytes within the QLT-0276-treated group. iO, immature oogonia; mO, mature primary oocytes; scale bars: 50 μm.
|
|
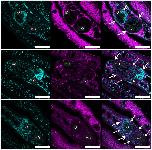
Fig 10. QLT-0276 affects the epithelium surrounding the ovary in paired females.Fluorescence microscopy of paired females with a focus on their ovaries. Females were treated for 3 days with DMSO (upper panel) or QLT-0276 (10 μM, middle panel; 100 μM, lower panel) before staining with Hoechst 33342 (left) or anti-α-laminin (middle). Merged images are shown right. Arrows indicate the α-laminin containing epithelium (upper) surrounding the ovary. In ovaries of treated females α-laminin staining was remarkably reduced or missing (dotted arrows). iO, immature oogonia; mO, mature primary oocytes; scale bars: 50 μm.
|
|
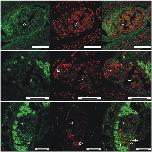
Fig 11. TUNEL-positive cells indicate apoptosis in oocytes of females treated with QLT-0276.Fluorescence microscopy to visualize the results of TUNEL assays performed with paired females treated for 3 days with DMSO (upper panel) or QLT-0276 (10 μM, middle panel; 100 μM, lower panel). Staining was performed by FITC-labeled dUTP (left) or propium iodide (middle). Merged images are shown right. Arrows indicate TUNEL-positive cells. iO, immature oogonia; mO, mature primary oocytes; scale bars: 50 μm.
|
|
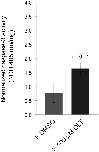
Fig 12. Caspase-3 activity increases following QLT-0276 treatment in paired females.Result of the caspase-3 activity assay showing that compared to a DMSO-treated control (left) the activity of caspase-3 of S. mansoni increased significantly (p = 0.01) in paired females treated with 100 μM QLT-0276 (right) as determined spectrophotometrically (ΔOD405).
|
|
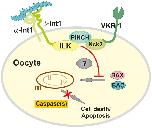
Fig 13. Hypothetical model of the function of the Smβ-Int1/SmVKR1 complex in mature S. mansoni oocytes.In paired S. mansoni females the complex formation of Smβ-Int1-SmILK-SmPINCH-SmNck2-SmVKR1 prevents cell death processes such as apoptosis mediated by the pro-apoptotic BCL-2 family members BAK and BAX and by caspases (red line, red cross). The process is probably induced by interaction of integrins (Smα/Smβ integrins) with the extracelluar matrix (blue trapezoids). Molecules mediating this effect from the membrane-located complex members to the cytosol are still unknown (?). m, mitochondrium.
|













